Most people think obesity is just about eating too much and moving too little. But if that were true, why do some people stay lean no matter what they eat, while others gain weight even on modest portions? The real story is deeper-it’s in your brain, your hormones, and the way your body fights to hold onto every extra calorie. Obesity isn’t a choice. It’s a medical condition rooted in broken biology.
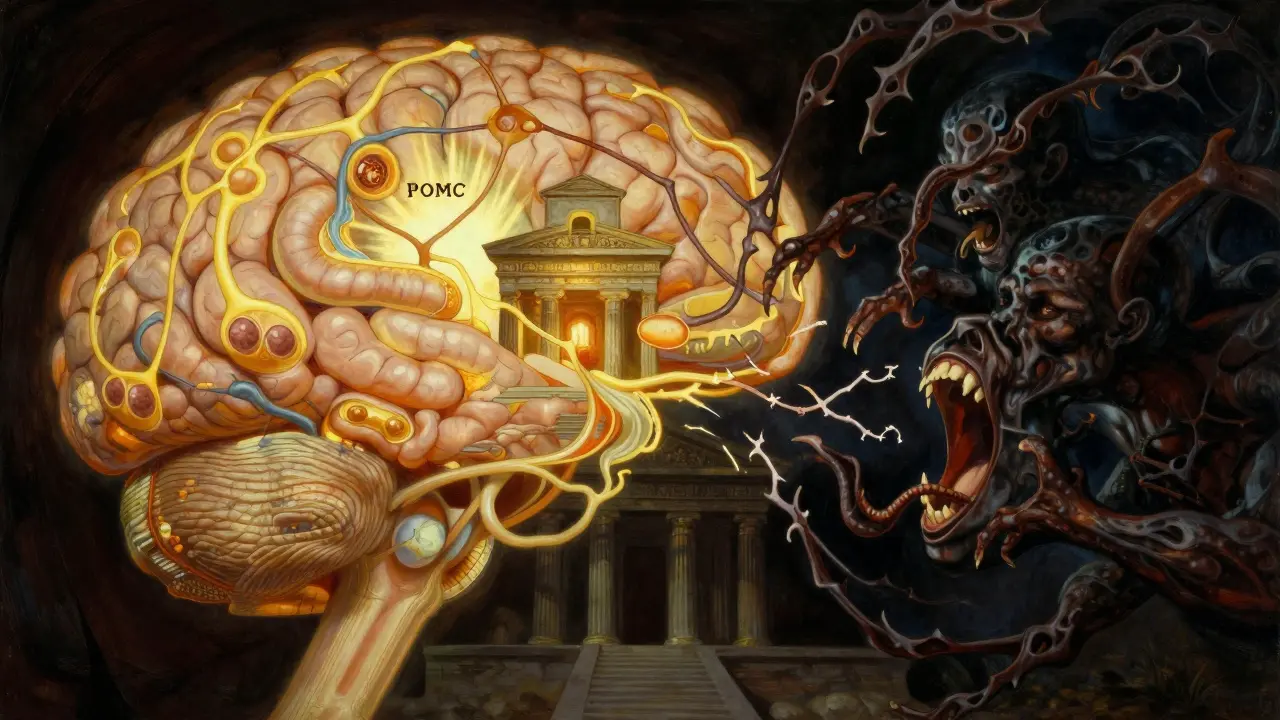
The Brain’s Hunger Switches Are Stuck
Your hypothalamus, a tiny region deep in your brain, acts like a thermostat for your body weight. Inside it, two sets of neurons are locked in constant battle. One group, called POMC neurons, tells you to stop eating. They release alpha-MSH, a signal that makes you feel full. The other group, NPY and AgRP neurons, screams for more food. They’re the ones that make you crave snacks at 2 a.m. or feel hungry again an hour after a big meal. In a healthy person, these systems balance each other. But in obesity, the hunger signals win. Studies show that when AgRP neurons are artificially turned on in mice, they eat 300 to 500% more food in minutes. That’s not willpower-it’s biology overriding reason.Leptin: The Fat Hormone That Stopped Talking
Your fat cells don’t just store energy-they talk. They release leptin, a hormone that tells your brain, “We’ve got enough.” In lean people, leptin levels sit between 5 and 15 ng/mL. In obesity, they spike to 30-60 ng/mL. You’d think that would shut down hunger, right? Wrong. That’s the problem. Your brain stops listening. This is called leptin resistance. It’s not that you don’t have enough leptin-it’s that your hypothalamus no longer responds to it. The signal is there, but the receiver is broken. This is the core issue in over 99% of obesity cases, not a lack of leptin. Only about 50 people worldwide have true leptin deficiency, and they’re the exception.Insulin, Ghrelin, and the Hormonal Rollercoaster
Leptin isn’t alone. Insulin, the hormone that manages blood sugar, also tells your brain to reduce appetite. After a meal, insulin rises from 5-15 μU/mL to 50-100 μU/mL. In healthy people, that drop in hunger is smooth. In obesity, insulin resistance in the brain blunts this effect. Your brain doesn’t get the message that you’re full. Then there’s ghrelin-the only known hunger hormone. It spikes before meals, from 100-200 pg/mL to 800-1000 pg/mL. In people with obesity, ghrelin doesn’t drop as it should after eating. That means your stomach keeps crying out for food, even when you’ve had enough. Some studies show ghrelin levels stay high longer after meals in obese individuals, making it harder to feel satisfied.
The Broken Pathways Inside Your Cells
It’s not just hormones. Inside your brain cells, the signals get tangled. Leptin and insulin both use the PI3K-AKT pathway to suppress appetite. When this pathway works, it reduces food intake by 30-50%. But in obesity, inflammation and excess fat trigger JNK, a stress pathway that blocks PI3K-AKT. It’s like someone jamming the brakes on your brain’s full signal. The mTOR system, which helps cells sense nutrients, also gets disrupted. When mTOR is activated, it reduces hunger. But in obesity, mTOR signaling becomes erratic. Some parts overreact, others shut down. This chaos makes it harder for your body to adjust to food intake. Even the BMP4 protein, which helps regulate appetite, becomes less effective. In obese mice, giving BMP4 cuts food intake by 20%. But in humans, this pathway doesn’t respond the same way-likely because the system is already overwhelmed.Why Diets Fail (It’s Not Your Fault)
When you lose weight, your body fights back. Leptin drops. Ghrelin rises. Your metabolism slows. Your brain interprets weight loss as starvation. That’s why most people regain the weight-even if they stick to the diet. A 2022 study found that after weight loss, the brain’s reward centers become more sensitive to high-calorie foods. A slice of pizza doesn’t just taste good-it feels essential. That’s not weakness. It’s evolution. Your brain is wired to survive famine, not a buffet. And then there’s pancreatic polypeptide (PP). This hormone, released after eating, slows digestion and reduces hunger. But in 60% of people with obesity, PP levels are abnormally low. That means your body doesn’t get the signal to stop eating-even after a full meal.




Written by Martha Elena
I'm a pharmaceutical research writer focused on drug safety and pharmacology. I support formulary and pharmacovigilance teams with literature reviews and real‑world evidence analyses. In my off-hours, I write evidence-based articles on medication use, disease management, and dietary supplements. My goal is to turn complex research into clear, practical insights for everyday readers.
All posts: Martha Elena